Why do we inject insulin, anyway?
There are – perhaps soon to be were – several reasons. For one, insulin breaks down during digestion. Insulin is a poly-peptide protein, but by the time it’s run the gauntlet of various enzymes and juices, it ends up being just a single peptide, which is a bit useless.
Even if it did work, it wouldn’t work well enough. Insulin has to be specifically measured to address the specifics of your blood glucose. Food, exercise, stress – there are a multitude of conditions that can affect your sugar levels.
Injected insulin gets straight into the blood with a minimum of fuss. It doesn’t breakdown or change much, because it goes straight into the subcutaneous fat. Insulin is carefully designed to work within a particular time; that’s why we have long-acting, rapid-acting, and lots of other-acting kinds of insulin. If insulin went straight into the bloodstream, it would be absorbed too quickly.
Insulin treatment is a carefully choreographed process. Only through injection does it function exactly as it’s supposed to.
Those of you who are up on your diabetes news will be yawning and rolling your eyes at my old-fashioned commitment to injected insulin. You know your Afrezza from your Exubera, and you know that effective alternative insulin treatments could be just ’round the corner.
But such developments aren’t there yet, and the paragraphs above explain why. These are the challenges facing manufacturers: we want to develop a more convenient form of insulin treatment, but how do we make it function meticulously?
Because injected insulin has its problems: for one thing, it’s uncomfortable – nobody likes stabbing themselves with needles, even if it is of life-prolonging importance; the process of overcoming needle phobia for some people with type 1 diabetes is a harrowing one; and insulin injections can make your skin lumpy and bruised.
Granted, things are better than they used to be – shortly after it was invented, insulin injections were akin to stabbing yourself with a knitting needle – but a less unpleasant alternative would be welcome.
There’s a rich history of alternative forms of insulin treatment.

6. Nasal insulin
Insulin up the nose isn’t ideal either. But it’s been tried. Its purpose was to deliver insulin directly to the brain. It was never really used as a treatment for type 1 diabetes. Rather, researchers were interested in it as a treatment for cognitive conditions like Alzheimer’s, which is strongly linked to brain damage caused by high blood glucose levels.

5. Exubera
Introduced in 2006, withdrawn in 2007 – Exubera was a nice idea. Unfortunately, it was enormous, which was problematic for two reasons: it was difficult to carry around, and it looked a bit weird, really.
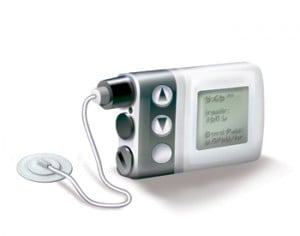
4. Insulin pumps
Insulin pumps are still a bit invasive, but they remove the need for all the self-stabbing. Instead, they’re attached using a catheter placed under the skin. Bolus insulin is delivered automatically, with users able to adjust when and how much.

3. Afrezza
Afrezza is a rapid-acting inhaled insulin. And, unlike the notorious Exubera, it’s really tiny, about the size of your thumb. It’s just a mealtime insulin, mind you; you’ll still need your basal injections. While Afrezza has been approved by US Food and Drug Administration, there’s no such good news for the UK market yet.

2. Artificial pancreas
The artificial pancreas isn’t quite there yet, but it’s a pretty inspiring idea. In theory, the artificial pancreas will detect low blood glucose levels and stop administering insulin in response. For a long time, the closest thing we’ve got is a combined insulin pump and blood glucose monitor – not the two devices wrapped together with masking tape like you’re imagining, but a separate device that combines both technologies.
In January, we saw a breakthrough, when four-year-old Xavier Hames was fitted with the first ever artificial pancreas. It’s a pump that knows when his blood glucose levels are low and stops delivering the insulin, without the need for him to adjust the pump manually. Problem is, the device costs more than £5,000.
Since then, a lady from Norfolk gave birth while using the artificial pancreas to a healthy baby.

1. Bionic pancreas
The bionic pancreas is even better. Like the artificial pancreas, the bionic pancreas can detect low blood glucose levels, at which point it will stop administering insulin. Unlike the artificial pancreas, the bionic pancreas can also administer glucagon, making it capable of treating hypoglycemia.
You have two pumps – one for insulin, and one for glucagon – and an iPhone. Through the use of Bluetooth technology, the two pumps can communicate via the iPhone and work out how much of each is needed, based on continuous glucose monitoring. To me it sounds like it could be used by the Terminator, but I’m easily baffled by technology.
The bionic pancreas is being developed by researchers at Boston University. The team hope to conduct the final clinical trial later this year, and have it on the market by late 2017.



