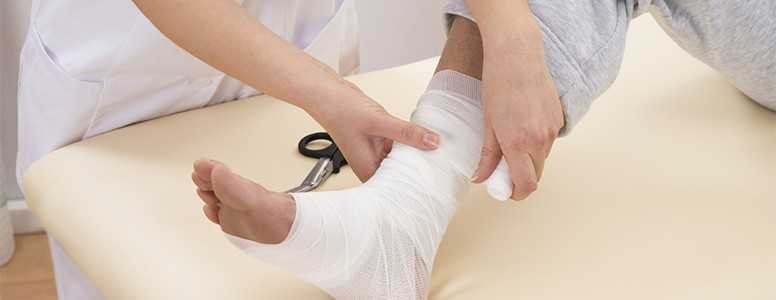
The US Food and Drug Administration (FDA) has warned that the type 2 diabetes drug Invokana (canagliflozin) could be associated with an increase in leg and foot amputations.
Invokana, an SGLT2 inhibitor, helps to lower blood glucose levels in type 2 diabetes patients by encouraging the body to filter out glucose from the blood and excrete it through urine.
The FDA’s warning was made following results of the ongoing Canagliflozin Cardiovascular Assessment Study (CANVAS) trial, which found amputations occurred roughly twice as often in patients treated with Invokana compared to those treated with placebo.
Patients in the CANVAS trial have been followed for an average of 4.5 years. An interim analysis found that the risks of amputation were equivalent to seven out of every 1,000 patients treated daily with 100mg canagliflozin; five out of every 1,000 patients treated daily with 300mg canagliflozin; and three out of every 1,000 patients treated with placebo.
However, the FDA noted it hasn’t been determined if cangliflozin increases the risk of amputations, rather a correlation has been found. Further research will be required to assess if the drug is responsible for the elevated risk, and the FDA has urged patients not to stop taking the drug in the meantime.
“Patients should not stop or change their diabetes medicines without first talking to their health care professional,” said the FDA. “Doing so can lead to uncontrolled blood sugar levels that can be harmful.”
Patients have also been advised to seek immediate medical attention if they experience any pain, tenderness, sores or ulcers in their legs or feet.
“We are continuing to evaluate this safety issue and will update the public when we have more information,” added the FDA.
What's new on the forum? ⭐️
Get our free newsletters
Stay up to date with the latest news, research and breakthroughs.