On February 12, 2016, the National Institute for Health and Care Excellence (NICE) published ‘Integrated sensor-augmented pump therapy systems for managing blood glucose levels in type 1 diabetes (the MiniMed Paradigm Veo system and the Vibe and G4 PLATINUM CGM system‘. In this 49-page document, NICE outlined recommendations to provide technology to certain people with type 1 diabetes to help them control their blood glucose levels.
We’ve trawled through the recommendations so you don’t have to. In this blog, we’ll answer the big questions surrounding the recommendations: Who is eligible? What exactly are they eligible for? Is it likely that the system will be made widely available in the future?
Who is eligible?
Prescription is subject to several conditions. To be eligible, you have to “have episodes of disabling hypoglycemia despite optimal management with continuous subcutaneous insulin infusion”. In other words, you must be regularly affected by severe hypoglycemia, but not because you have poorly controlled blood glucose levels. The guidelines only cover people who generally look after their blood glucose levels very well, but nevertheless experience severe hypos on a regular basis.
Optimal management means keeping HbA1c levels within the target range, indicating a generally meticulous attitude to diabetes management.
There are other conditions, too. You must agree to use the sensors for at least 70 per cent of the time, understand how to use the system, and agree to follow a structured education programme at the same time. The programme covers diet, lifestyle and counselling. In addition, the company prescribing the MiniMed Paradigm Veo system must agree to collect, analyse and publish your usage data, but that doesn’t affect you so much.
To keep being given access to the system, you have to show a sustained reduction in episodes of hypoglycemia. So, if you stay the same or get worse, it’s not worth you continuing. Targets for hypo reductions are agreed with your doctor.
What are they eligible for?
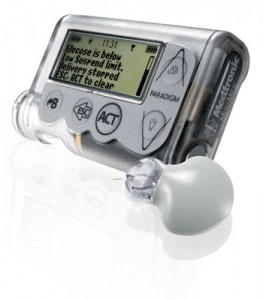
The document evaluates two systems. One is called the MiniMed Paradigm Veo system; the other is called the Vibe and G4 PLATINUM CGM system. Both systems combine insulin pump therapy with continuous glucose monitoring.
The MiniMed Paradigm Veo has three components. There is a sensor placed under the skin, which constantly monitors blood glucose levels through the interstitial fluid. There’s also an insulin pump, which delivers insulin to the subcutaneous tissue (the bit of fat where insulin is injected). Finally, there’s the MiniLink non-implanted transmitter, which sends readings from the sensor to the pump.
When blood glucose levels run low, the Veo system plays an alarm sound. One of the Veo’s most important features is called a “low-glucose suspend function”. When blood glucose levels run too low, the Veo automatically stops the pump’s insulin delivery for two hours, all the while signalling to the user that blood glucose levels are low.
Like the Veo, the G4 PLATINUM CGM is made up of three parts: a pump, a sensor and a transmitter. It provides warnings and alarms when blood glucose levels run low, but it does not automatically suspend insulin delivery.
The authors express a hope that “integrated sensor-augmented pump therapy systems may improve glucose control and consequently may reduce the number of diabetes-related complications and improve the quality of life for people with type 1 diabetes.” It is also hoped that both systems “offer benefits to the NHS through cost and resource savings by reducing the number of hospital admissions and consultations for diabetes-related complications, and by achieving optimum blood glucose control more quickly.”
Is this CGM technology likely to be made widely-available?
The guideline is a positive move for people with type 1 diabetes on insulin pump therapy. Studies have already shown that the MiniMed Paradigm Veo has the ability to reduce the impact of hypos, particularly overnight hypos.
Use of the technology, under the new guidance, will also allow the NHS to build up evidence of how effective the CGM sensors are in preventing severe hypos in day to day use.
The guidelines show that NICE is looking to roll out the new technology carefully. At first, the guidelines will make the technology available to only a small proportion of people with type 1 diabetes in order to collect data on effectives. But, if it’s successful, the technology may be more widely available soon.
NICE is responsible for ensuring new treatments and technology are cost-effective, and these guidelines are an important first step towards verifying how effective sensor-augmented insulin pump therapy can be.
Featured image source: animascorp.co.uk





