Cellnovo, a diabetes technology company based in Bridgend, Wales, has filed for 510(k) approval with the US Food and Drug Administration (FDA) for its mobile, all-in-one diabetes management system.
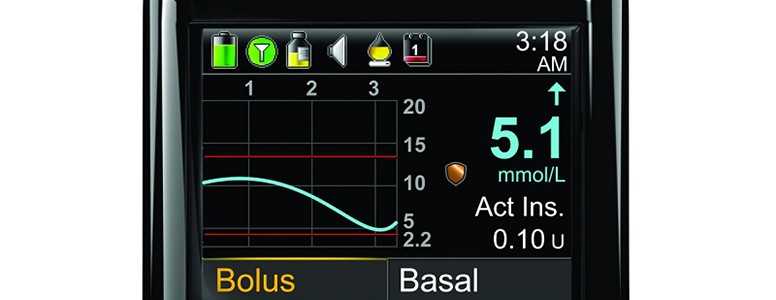
The all-in-one diabetes management system combines an insulin pump, a touchscreen handset that functions as both a blood glucose meter and controls the pump, and mobile apps that allow you to analyse your control and treatment in detail.
Because it is controlled by the handset, the Cellnovo insulin pump is very small and can be worn as a tubeless patch pump. This has advantages such as reducing problems with bubbles and preventing tubing-related problems such as tubing getting caught on door handles or hampering performance in sports.
The 510(k) approval from the FDA is needed before the system can be launched in the USA. The approval process is expected to take several months.
If successful, it will be a great step forwards for Cellnovo, a company from Wales.
Chief Executive Officer of Cellnovo, Sophie Baratte stated: “We are pleased to have submitted our application to the US authorities. The United States represents an important future market for Cellnovo with 1.25 million children and adults with type 1 diabetes.
“Furthermore, market penetration of insulin pumps is higher in the US than anywhere else in the world. We believe that our diabetes management system, offering unrivalled levels of freedom and connectivity combined with high levels of accuracy, has the potential to perform strongly in the growing insulin pump marketplace.”
The Cellnovo System is available on the NHS for those meeting insulin pump eligibility.
What's new on the forum? ⭐️
Get our free newsletters
Stay up to date with the latest news, research and breakthroughs.