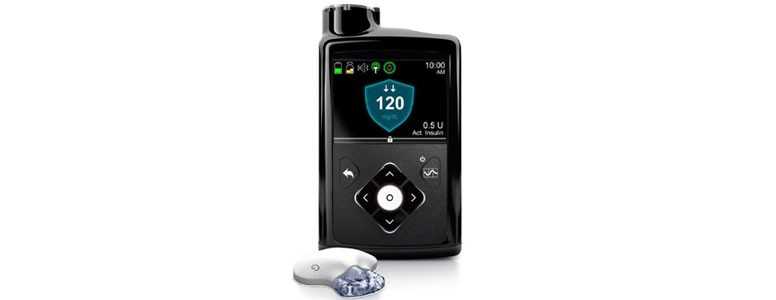
A “first-of-its-kind” hybrid closed loop system has been approved in the US to help people living with type 1 diabetes achieve much greater freedom.
The life-changing MiniMed 670G, produced by Medtronic, has been approved by the US Food and Drug Administration (FDA).
The device, which pairs insulin pump and continuous glucose monitoring (CGM) technology, monitors glucose levels and provides appropriate basal insulin doses in people 14 years of age and older with type 1 diabetes.
Dr Jeffrey Shure, director of the FDA’s Center for Devices and Radiological Health, said: “This first-of-its-kind technology can provide people with type 1 diabetes greater freedom to live their lives without having to consistently and manually monitor baseline glucose levels and administer insulin.”
A working human pancreas naturally supplies a low, continuous rate of insulin, known as basal or background insulin. However, in people with type 1 diabetes, the body lacks the ability to produce its own insulin.
The MiniMed 670G hybrid closed looped system works by measuring glucose levels every five minutes and can automatically respond by administering or withholding insulin.
The system is the closest system to being an artificial pancreas that has been approved to date. The system’s ability to respond to glucose levels by increasing or decreasing rates of insulin is a key step forwards. Users of the system will still need to program in bolus insulin doses around meals.
A clinical trial of 123 participants with type 1 diabetes showed the device is safe and no serious adverse events, such as diabetic ketoacidosis (DKA) or severe hypoglycemia (low glucose levels) were reported.
Medtronic is now performing clinical studies to evaluate the safety and effectiveness of the device in children with diabetes aged between seven and 13.
What's new on the forum? ⭐️
Get our free newsletters
Stay up to date with the latest news, research and breakthroughs.