A pivotal trial of the diabetes closed loop system will try out a new sensor, designed by the American company Senseonics, as part of a partnership with TypeZero and Roche Diabetes Care.
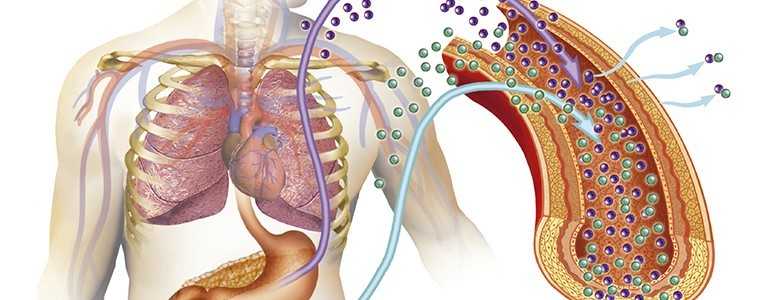
The International Diabetes Closed Loop (iDCL) trial, which is ongoing, will test an artificial pancreas (AP) system for the management of type 1 diabetes integrating Senseonic’s latest continuous glucose monitoring (CGM) technology Eversense.
Eversense will be paired to Roche’s Accu-Chek Insight pump and TypeZero’s inControl AP algorithm, which will automatically adjust and regulate insulin based on data received from the new CGM and the insulin pump.
The collaboration is part of a National Institutes of Health (NIH)-funded research project. In the lead up to it, Roche committed to help Senseonics raise $41 million to make the Eversense technology AP system-ready.
This hybrid AP system for use in the iDCL trial is built around Eversense’s fully implantable sensor, which once in place uses fluorescence technology to measure blood sugar levels in interstitial fluids of the upper arm.
The glucose-responsive sensor, which lasts up to 90 days, sends readings to a smart transmitter that processes the data and relays information to a smartphone app which can alert users when Eversense forecasts glucose levels will exceed preset ones.
Most of the iDCL trial will take place at the Centre for Diabetes Technology of the University of Virginia. Three European research centres are also set to test this new version of the closed loop AP system.
These will include the University of Amsterdam in the Netherlands, the University of Padova in Italy and the University Hospital of Montpellier, in France.
This collaboration within the iDCL trial between TypeZero, Roche and Senseonics holds promise for enhancing people’s time in range as well as reduce the occurrence of hypoglycemia.
What's new on the forum? ⭐️
Get our free newsletters
Stay up to date with the latest news, research and breakthroughs.