Phase III results of the CANTOS heart trial have shown that a drug, previously tested in type 2 diabetes, cuts cardiovascular disease (CVD) events.
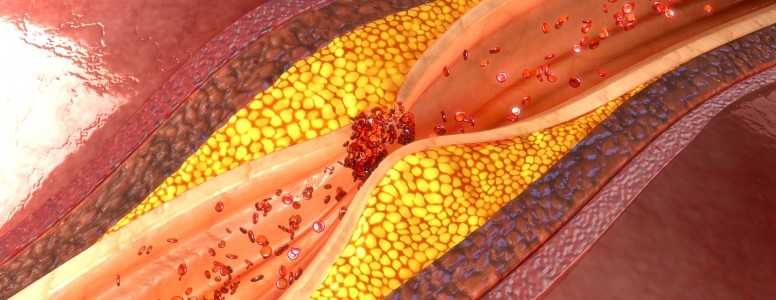
The drug in question is canakinumab (ACZ885), an antibody to a pro-inflammatory cytokine molecule, called Il-1B, which is known to drive atherosclerosis. Atherosclerosis is the name for the damage to blood vessels whereby arteries become clogged.
Canakinumab is the first and only agent which has shown that selectively targeting inflammation significantly reduces CVD risks.
In the six-year trial, drug maker Novartis tested canakinumab plus standard of care in 10,061 patients who had previously suffered a myocardial infarction (MI).
The participants all had a high level of inflammation in the body, as evidenced by elevated levels of high-sensitivity C-reactive protein (CRP).
Previous research suggested that high CRP levels may be a reliable indicator of atherosclerosis and that it can influence risks of having heart problems return.
The inflammation hypothesis of atherosclerosis holds that plaque appear to repair damaged blood vessels that have been exposed to the forces of inflammation and oxidative stress.
The CANTOS trial represents an important breakthrough in cardiovascular medicine as it shows for the first time that reducing inflammation through Il-1B reduces CVD risks.
The Phase III CANTOS study showed that canakinumab met its primary endpoint in increasing the time to a heart attack, stroke or CVD death compared with standard of care alone.
Although full results will not appear until later this year, the preliminary data support the hypothesis that inflammation plays at least some direct role in atherosclerosis.
What's new on the forum? ⭐️
Get our free newsletters
Stay up to date with the latest news, research and breakthroughs.