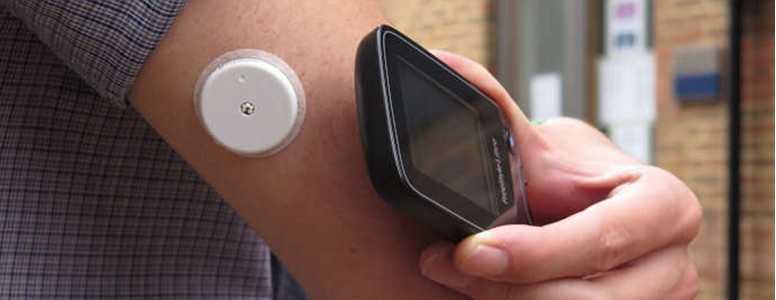
Abbott, the company that makes the FreeStyle Libre, has said production of the device will increase by three to five times in the next few years.
Manufacturing of the FreeStyle Libre, a flash glucose monitoring system, is to be ramped up so millions more people around the world will have access to it.
Earlier this year the NHS announced detailed plans for increased FreeStyle Libre access in the UK, so that the device will be available for one in five people with type 1 diabetes.
Abbott’s senior vice president for Diabetes Care, Jared Watki, said up-scaling production was a focal point for the company’s strategy for its glucose monitors.
From the moment the FreeStyle Libre was made available in Europe in 2014 it has received acclaim for its positive impact on people with type 1 diabetes. A UK study this year revealed the device reduced incidences of low blood glucose in roughly 80% of people with type 1 diabetes.
The Libre works via connection to a sensor attached to the user’s arm, allowing them to track glucose levels throughout without the requirement for multiple daily finger sticks (although intermittent blood glucose tests are still recommended).
The system is currently being used by one million people with type 1 diabetes and half a million with type 2 diabetes across 44 countries. But with more people being diagnosed with type 2 diabetes and the FreeStyle Libre offering freedom of needles for those with type 1 diabetes, demand is expected to increase.
Mr Watkin added: “The need to invest and bring up capacity is, we believe, going to be an ongoing activity for us.”
The announcement comes as the company is set to launch the FreeStyle Libre 2 in the US. The updated device has already been approved for use in Europe.
The next-generation FreeStyle Libre has more features, including alarms that alert the user when blood glucose levels go too low or high. Despite that, it will remain the same price as the current version.