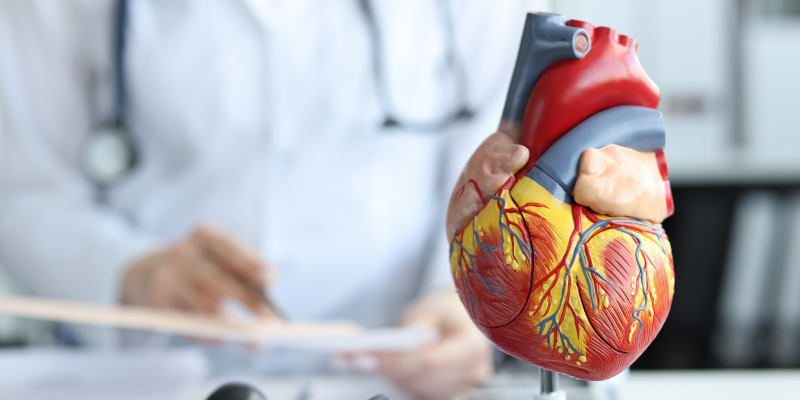
The structure of the heart’s ventricles has been recreated by bioengineers, bringing them one step closer to creating an artificial heart for transplant.
The American team have developed a model of ventricles which replicates the muscle alignment that is crucial to ensuring enough blood is pumped with every contraction.
Creating the spiralling muscles found in the heart leads to the twisting motion that occurs when the heart beats – something that experts have said is critical for pumping large amounts of blood.
Dr Kit Parker, the Tarr Family Professor of Bioengineering and Applied Physics at Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), said: “This work is a major step forward for organ biofabrication and brings us closer to our ultimate goal of building a human heart for transplant.”
The heart cannot repair itself like other organs do, and with heart disease the most common cause of death in America, the development of an artificial heart is crucial.
Bioengineers have been working to recreate the unique structures which make up the heart, and have now proved that the spiral shape of the muscles does boost the volume of blood that can be pumped.
The spiralling muscles was first noted in 1669 by an English doctor, Richard Lower, and since then, their function has been studied in depth to try and understand their purpose, but studies have proved difficult.
Edward Sallin, former chair of the Department of Biomathematics at the University of Alabama Birmingham Medical School, said in 1969 that the arrangement of the heart’s muscles and its helical alignment is crucial to how much blood can be pumped.
The team from SEAS used the latest technology to build a model which tested this theory. Using a Focused Rotary Jet Spinning system, which works like a candy floss machine, they were able to spin fibres which they could grow cardiac cells on. The spinning system allowed the team to twist and align the fibres, replicating the helical structure of heart muscles.
Dr Parker said: “Since 2003, our group has worked to understand the structure-function relationships of the heart and how disease pathologically compromises these relationships.
“In this case, we went back to address a never tested observation about the helical structure of the laminar architecture of the heart. Fortunately, Professor Sallin published a theoretical prediction more than a half century ago and we were able to build a new manufacturing platform that enabled us to test his hypothesis and address this centuries-old question.”
The study has been published in the journal Science.